Abstract
BACKGROUND
The role of autologous stem cell transplantation (ASCT) in Waldenström Macroglobulinaemia (WM) is not well established, largely due to the paucity of evidence. It remains unclear where ASCT should be placed in the sequence of treatment lines. The debate is stronger in the era of targeted therapies that can achieve prolonged progression free survival (PFS) intervals and expected treatment-free intervals of approximately 4 to 8 years. The goal of this real world analysis was to review response and survival outcomes, and relapse risk factors in WM patients who underwent ASCT in a single WM referral centre.
METHODS
A retrospective cohort analysis was undertaken of consecutive WM patients treated with ASCT at a single specialist centre between 2003 and 2020. Baseline demographic/biological data, number/types of prior therapies, and pre- and post-ASCT depth of response, were collected from patient records and the WMUK Rory Morrison Registry. The primary aims were to determine depth of response, overall survival (OS), progression free survival (PFS), transplant related mortality (TRM) and relapse-associated mortality.
RESULTS
A total of 32 patients received ASCT, with a median age at time of ASCT of 57 years (range 40-68 years) and median interval from diagnosis to ASCT of 2.3 years (range 0.5-16.8 years); 14 (43.7%) were male. Prior to ASCT, 11 patients (34%) had received one therapy, 11 patients had 2 lines of treatment (excluding mobilisation), and 10 patients (31%) had received 3 or more therapies. The disease status pre-ASCT was complete remission (CR)/very good partial response (VGPR) in 14 patients (43.7%) and partial response (PR) in 18 patients (56.2%).
Conditioning therapy comprised LEAM (Lomustine, Etoposide, Cytarabine, Melphalan; 18 patients, 56.2%), BEAM (Carmustine substituted for Lomustine; 12 patients, 37.5%), or Melphalan only (2, 6.2%). All patients had successful engraftment. Median time from stem cell reinfusion to hospital discharge was 15.5 days (range 13-187 days) in the 24 patients for whom these data were available; 5/24 patients (21%) were discharged >25 days after stem cell reinfusion. Restaging at 100 days post-ASCT showed deepening of response by ASCT in 17 patients (53.1%). CR/VGPR was achieved by 26 patients (81.2%) and PR by 4 patients (12.5%). Two patients (6.2%) experienced disease progression before day 100 post-ASCT (both receiving ASCT in second remission/PR2).
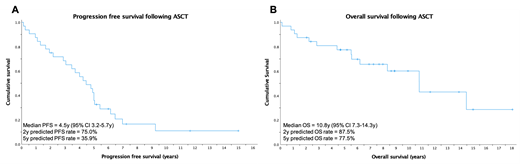
At a median follow up of 8.9 years (range 0.1-18 years), the estimated median PFS was 4.5 years (95% confidence interval [CI] 3.2-5.7 years), with estimated 2-year and 5-year PFS rates of 75% and 35.9%, respectively (Figure 1A). In this small cohort, there was no significant difference in PFS based on age, number of prior lines of treatment, pre-ASCT remission status (CR/VGPR vs PR) or post-ASCT response achieved.
At time of analysis, 14/32 patients (43.7%) had died: TRM rate was 6.2% (2 patients died during inpatient admission of ASCT complications), 4 patients (12.5%) died of PD, and 1 patient died of unknown causes. Another 7 patients (21.9%) died of infective causes after the immediate post-ASCT period: the median time from ASCT to death among these patients was 5.5 years (range 0.8-10.8 years). Estimated median OS for the unstratified cohort was 10.8 years (95% CI 7.3-14.3 years), with estimated 2- and 5-year OS rates of 87.5% and 77.5%, respectively (Figure 1B). Overall survival did not differ significantly based on age at time of ASCT, number of therapy lines prior to ASCT, pre-ASCT remission status (CR/VGPR vs PR) or post-ASCT response achieved.
One patient (3.1%) underwent ASCT after BTK inhibitor therapy, achieving deepening of response (PR to VGPR) with ASCT and progression free interval of 11 months.
CONCLUSION
ASCT is a feasible treatment option for patients with relapsed WM, producing deeper responses following salvage therapy and resulting in PFS intervals comparable to other currently available therapeutic options. With the benefit of a long follow up period, performing ASCT at later stages in the treatment course (i.e. following 3 or more prior therapy lines) did not appear to result in inferior survival outcomes; timing of ASCT should therefore be considered on an individual patient basis and in light of other available therapy options for relapsed disease.
Yong: Sanofi: Honoraria, Research Funding; GSK: Honoraria; Amgen: Honoraria; Autolus: Research Funding; BMS: Research Funding; Janssen: Honoraria, Research Funding; Takeda: Honoraria. Wechalekar: Amgen: Research Funding; Alexion, AstraZeneca Rare Disease: Consultancy; Caelum Biosciences: Other: Clinical Trial Funding; Takeda: Honoraria; Celgene: Honoraria; Janssen: Consultancy. D'Sa: Sanofi: Honoraria; Janssen Cilag: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal